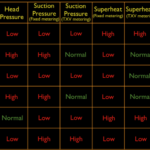
These terms describe essential operating parameters in refrigeration and heat pump systems. Saturated refers to the “phase” state of refrigerant: vapor or liquid or combination of the two. Refrigerant in a closed system can only exist as a superheated vapor or subcooled liquid or a saturated mixture of vapor and liquid.
Saturated conditions for all intents and purposes, is simply the boiling and condensing point of the vapor/liquid mixture. A drum of 410A sitting in your truck at a constant temperature contains 410A in a saturated condition. The liquid contains the maximum amount of heat possible without boiling and the vapor contains the least amount of heat possible without condensing. If some heat is added to the drum, some of the liquid will vaporize. If the drum loses any heat, some of the vapor will condense.
When a system is operating, the evaporator coil contains some amount of liquid that is flowing through the tubing at the liquid boiling point for the particular operating pressure. As it takes in heat energy from the airflow, that heat is consumed as “latent heat of vaporization” which doesn’t change the liquid temp; it creates the phase change to vapor.
Once all the liquid has vaporized, the remaining vapor will continue to take in heat as a sensible process and increase in temperature. The increase in temperature is referred to as superheat. “Super” in this case, simply means “above.” The vapor has increased some amount greater than then saturated temperature you are reading on the pressure gauge. If the gauge reading is 45˚ saturated and the vapor line measures 55˚, the vapor temp has increased 10˚.
The same logic, sort of in reverse, applies to the condenser section. The vapor coming from the compressor is superheated above the condensing temp. On entering the condenser coil, the hot vapor will begin to lose heat to the outdoor air passing through the coil. It eventually cools down to the saturated temperature corresponding to the high side pressure value. At that point the vapor begins the phase change, condensing to the liquid state. At some point, all the vapor has condensed to the saturated liquid state. But it continues to give up heat to the outdoor air,” again as a sensible process, and decreases in temperature. The reduction in heat is referred to as “subcooling” with “sub” in this case meaning “below”. If the saturated condensing temp on the gauge shows 100˚ and the liquid line measures 90˚, the subcooling is 10˚.
It ain’t rocket science by any means but the numbers are critical when evaluating system operation. If the superheat or subcooling is running 2˚ or 3˚, something is wrong. If either is running 30˚, something is wrong. And to effectively evaluate or diagnose a system, one has to understand what the numbers mean.


Trying out 999jllogin for the first time. Registration was quick which is a good sign. Let’s see what the games are like! 999jllogin
Downloading the 9apisoapp now. Hopefully, it’s nice and optimized for mobile. Nothing worse than a laggy app when you’re trying to win! 9apisoapp
Checking out betmasters for some sports betting action. Hope they have good odds! Gotta do my research before I put any money down. betmasters